Prenylation covers two post-translational modifications in which one or two isoprenoid moieties, either farnesyl or geranylgeranyl moieties, are attached to a conserved cysteine residue via thioether bond at or near the C-terminus. Most prenylated proteins contain a CAAX motif at the C-terminus. The CAAX motif is a sequence of four amino acids, where “C” stands for a cysteine residue, “A” for an aliphatic amino acid and “X” for any amino acid1–4. The last amino acid “X” determines whether the protein is farnesylated or geranylgeranylated. Proteins with a C-terminal serine, methionine, alanine, or glutamine become farnesylated while C-terminal leucine becomes geranylgeranylated. However, there are few expections1. After prenylation the three C-terminal amino acids (AAX) are then cleaved off by RAS-converting CAAX endopeptidase 1. In addition, the prenylated cysteine is methylated by isoprenylcysteine carboxylmethyltransferase. Therefore, mature prenylated proteins possess at the C-terminus a farnesyl- respectively geranyl-geranylmethylcysteine1–4.
Prenylation is essential in maturation and processing of proteins. The individual sequence as well as the prenylated motif determine the localization of the protein. The prenylation of proteins strongly influences their properties. In addition to the localization, the modification also influences the stability of the protein and specific protein-protein interactions2. The addition of the isoprenoid group at the C-terminus causes a significant increase in hydrophobicity, which increases the capacity to interact with membrane proteins. More than 100 human proteins contain the CAAX motif and are thus potential substrates for prenylation1.
Farnesylation
Overview
Farnesylation is a subspecies of prenylation in which a 15-carbon isoprenoid lipid is attached to a cysteine residue by farnesyltransferase. Most prenylated proteins contain a CAAX motif at the C-terminus.
| pKa | NC | Loss | Gain | Deltamass | H | AA | UV-Spec | Pattern |
| – | No | H | C15H25 | Av: 204.3516 M: 204.1878 | – | C | – | C(?= [AILMFPWYV] {2}[AMSQ]) |
In-depth mechanism
Farnesol is a 15-carbon isoprenoid. The transfer of a farnesyl moiety is catalysed by farnesyltransferase. In order for the farnesyltransferase to be able to make the transfer, farnesol must be present in its active form as farnesyl pyrophosphate. During the modification, pyrophosphate is cleaved off and the farnesyl moiety is covalently bound to the cysteine residue of the CAAX motif1–4. Farnesylation increases the molecular mass by 204 Da5,6. The mechanism of farnesylation is shown in figure 1.
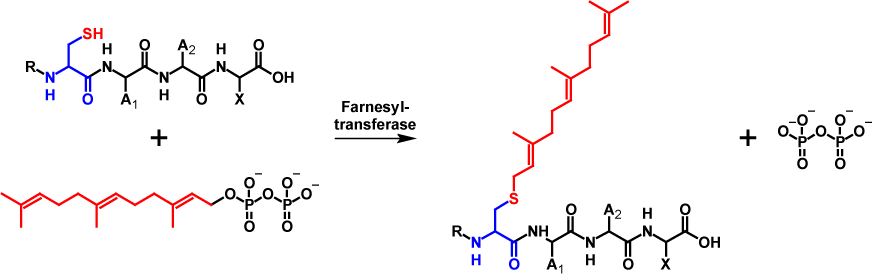
Geranylgeranylation
Overview
Geranylgeranylation is a subspecies of prenylation in which a 20-carbon isoprenoid lipid is attached to a cysteine residue by geranlygeranyltransferase I. Most prenylated proteins contain a CAAX motif at the C-terminus.
| pKa | NC | Loss | Gain | Deltamass | H | AA | UV-Spec | Pattern |
| – | No | H | C20H33 | Av: 272.4688 M: 272.2504 | – | C | – | C(?=[AILMFP WYV]{2}L) |
In-depth mechanism
Similar to farnesylation, geranylgeranylation involves attaching a 20-carbon isoprenoid to a cysteine residue of a protein that contains a C-terminal CAAX motif. The reaction is catalysed by geranylgeranyltransferase I. Analogue to farnesylation, during the reaction a geranylgeranyl moiety is covalently attached to the cysteine residue of the CAAX motif while pyrophosphate is released1–4. Geranylgeranylation increases the molecular mass of the protein by 272 Da6. The mechanism is shown in figure 2.
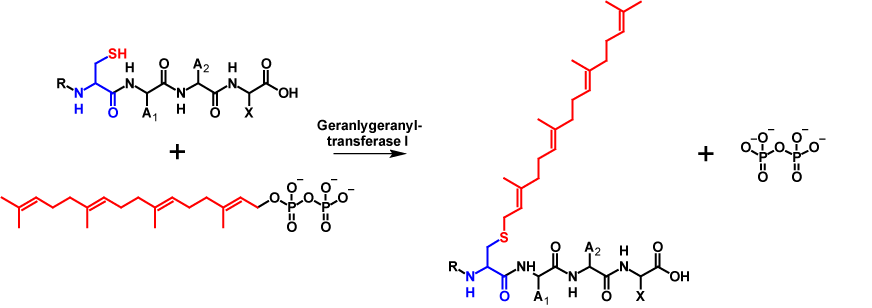
For geranylgeranylation, a CAAX motif does not necessarily have to be present. Geranylgeranyl-transferase II recognises Rab (member RAS oncogene family) proteins with a CC, CXC, CCX,CCXX, CCXXX or CXXX termination sequence. This makes a double geranylgeranylation possible. Methyl esterification only occurs on CXC motifs4.
- 1.Novelli G, D’Apice MR. Protein farnesylation and disease. Journal of Inherited Metabolic Disease. 2012;35:917–926. doi:10.1007/s10545-011-9445-y
- 2.Wang M, Casey PJ. Protein prenylation: unique fats make their mark on biology. Nature Reviews Molecular Cell Biology. 2016;17:110–122. doi:10.1038/nrm.2015.11
- 3.Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nature Chemical Biology. 2006;2:584–590. doi:10.1038/nchembio834
- 4.Roosing S, Collin RWJ, den Hollander AI, Cremers FPM, Siemiatkowska AM. Prenylation defects in inherited retinal diseases. Journal of medical genetics. 2014;51:143–151. doi:10.1136/jmedgenet-2013-102138
- 5.Heilmeyer LM, Serwe M, Weber C, Metzger J, Hoffmann-Posorske E, Meyer HE. Farnesylcysteine, a constituent of the alpha and beta subunits of rabbit skeletal muscle phosphorylase kinase: localization by conversion to S-ethylcysteine and by tandem mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9554–9558. doi:10.1073/pnas.89.20.9554
- 6.Bhawal RP, Shahinuzzaman ADA, Chowdhury SM. Gas-Phase Fragmentation Behavior of Oxidized Prenyl Peptides by CID and ETD Tandem Mass Spectrometry. Journal of The American Society for Mass Spectrometry. 2017;28:704–707. doi:10.1007/s13361-016-1503-0